‘Scared about smiling’: Suboxone's dental side effects spur nationwide class-action lawsuit
Suboxone is widely prescribed to treat opioid use disorder. Suboxone users are claiming the drug's producers failed to warn them of the risks
By Alexandra Keeler
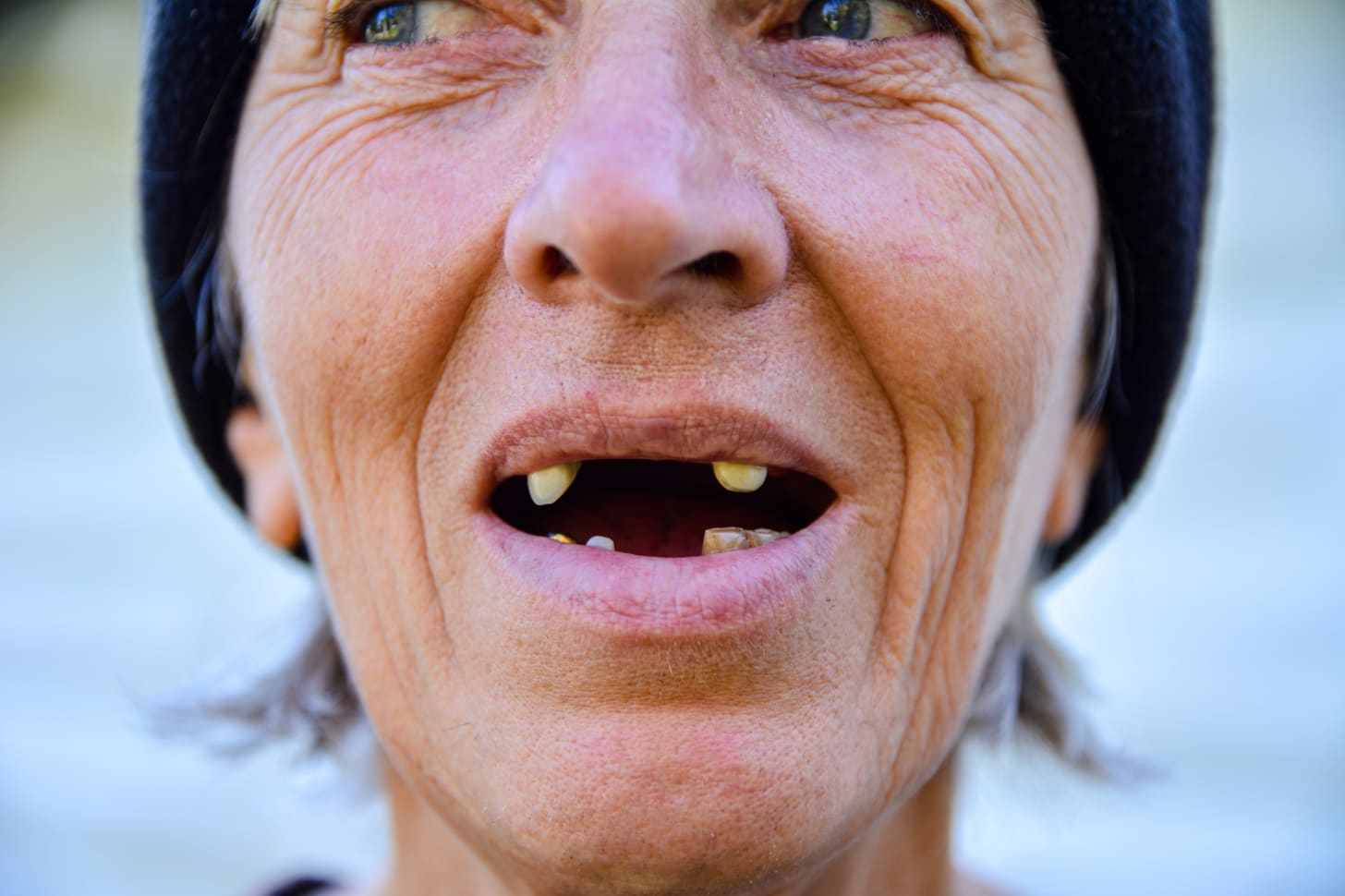
Gordon James Bull, 61, lost half of his lower teeth and a third of his upper teeth within two years of starting Suboxone, a drug prescribed for his chronic arthritis pain but which is commonly used to treat opioid use disorder.
Since the Squamish, B.C., resident began taking the drug a decade ago, Bull’s dental health has deteriorated so drastically he has had to undergo invasive dental procedures at substantial out-of-pocket expense. He also suffers from anxiety, stress and disturbed sleep over his dental condition.
Now, Bull is the lead plaintiff in a national class-action lawsuit against Indivior Canada Ltd., Indivior PLC and other entities that manufacture, market and distribute Suboxone.
“Something that I was not even previously aware of is the level of stigma and social anxiety that is caused to people when their teeth start to fall out or are jagged,” said Mohsen Seddigh, a partner at Sotos LLP who is representing Bull in the case.
“People start being scared about smiling.”
Indivior PLC has said it plans to contest the allegations.
“The Group has begun its evaluation of the claims, believes it has meritorious defenses, and intends to vigorously defend itself," said Jonathan Sibun, senior managing director at Teneo, a communications advisory firm that is representing Indivior.
'Fall through the cracks'
As Canada has grappled with a severe opioid crisis, the use of Suboxone and similar drugs has risen dramatically.
From 2015 to 2020, the number of Canadians receiving prescription opioids such as Suboxone for opioid-use disorder increased by 44 per cent, according to the Canadian Centre on Substance Use and Addiction, a non-governmental organization. By 2020, almost 4.4 million individuals received prescription opioids for opioid-use disorder.
Bull did not have a substance abuse issue, Seddigh says. But he anticipates that other plaintiffs in the lawsuit will. Class-action lawsuits need to receive court certification to proceed to a hearing on the merits. The certification process determines who is eligible to participate in the lawsuit.
“I typically deal with marginalized, vulnerable individuals,” Seddigh said. “There is a bigger tendency for them to fall through the cracks and go unnoticed because they're in no position to defend themselves.”
The lawsuit, which was filed in the Supreme Court of British Columbia in late May, alleges Indivior and related entities failed to adequately warn users about Suboxone’s dental side effects. In Bull’s case, these effects have included tooth loss, loss of fillings and tooth decay.
Suboxone must be dissolved under the tongue or in the cheek to be effective. The claim asserts this method of consumption increases mouth acidity, which leads to severe dental damage.
A 2023 study found that formations of the medication buprenorphine (which is used in Suboxone) that needed to be dissolved in the mouth were associated with significantly higher reports of dental problems than other formations of the medication.
Suboxone was approved by Canadian regulators in 2007. Suboxone’s product information guide did not include warnings about possible severe dental side effects until March 2023. The lawsuit alleges this change was made following pressure from the U.S. Food and Drug Administration.
Suboxone’s current product information guide warns users that use of the drug has been associated with dental issues, but notes that “causality isn’t definitively established.” It recommends regular checkups, rinsing the mouth after Suboxone dissolves and brushing one’s teeth after a waiting period.
Seddigh says prescribing physicians were not even aware of the drug’s risks.
“This disclosure [of adverse effects] is very important,” he said. “Giving the people the dignity and the right to choose for themselves.”
Indivior highlights patient safety on their website and advises individuals to report product concerns via email. Seddigh says he currently lacks details about Indivior's reporting mechanism and its purpose, which he hopes to access through litigation.
Bull’s case is not the first of its kind.
“I'm aware of a couple of similar cases in Canada,” says Seddigh.
One such case, which was filed in the Supreme Court of British Columbia in April, involves two B.C. residents who were prescribed Suboxone for pain management and opioid addiction. Their lawsuit, which is also a proposed class action against Indivior and related entities, also claims the companies failed to provide adequate warnings about potential dental issues.
Seddigh says his concern is with affording protections to vulnerable populations.
“When you're dealing with more vulnerable groups, there should be an added layer of protection,” he said. “But my experience has been that often there's fewer protections. If someone's living on the street, who are they going to complain to?”
[This article was produced through the Breaking Needles Fellowship Program, through which Canadian Affairs, an online media outlet, co-produces and co-publishes journalistic work on addiction and crime with the Centre For Responsible Drug Policy]